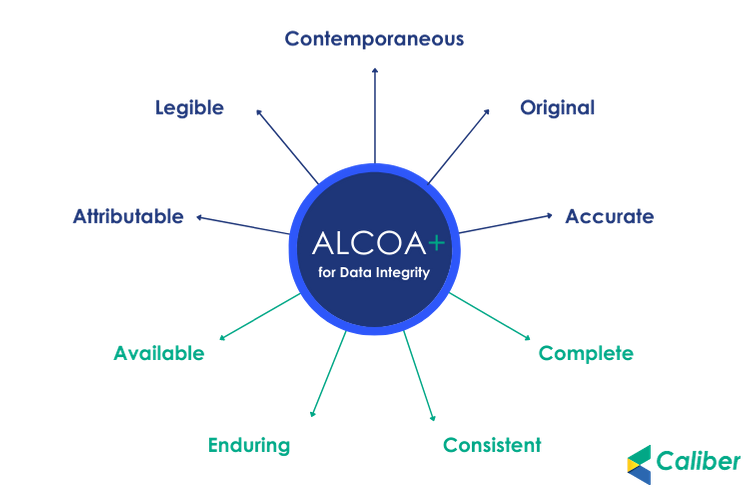
ALCOA is a set of principles that ensures data integrity in the life sciences sector It was introduced by and is still used by the FDA the US Food and. Since data integrity has become a hot topic in the pharmaceutical industry ALCOA has been cited as the ultimate reference. The criteria that define data integrity To meet regulatory requirements your laboratory data must be. The term ALCOA is an Acronym which stands for Attributable Legible Contemporaneous Original and Accurate ALCOA was then expanded to ALCOA. ALCOA is a set of principles and guidelines used in the life sciences and other regulated spaces The acronym which has expanded over the years..

Alcoa And Alcoa Plus Principles For Data Integrity Pharmaguideline
Ensuring Control of Chromatographs Integration and Results Register now for ECAs GMP Newsletter Since data integrity has become a hot. Seit Datenintegrität in der pharmazeutischen Industrie ein heißes Thema geworden ist wird ALCOA als ultimative Referenz angeführt. ALCOA is a set of principles and guidelines used in the life sciences and other regulated spaces The acronym which has expanded over the years. ALCOA is the extended form of ALCOA to further enhancement of data integrity The full form of ALCOA is Attributable. The criteria that define data integrity To meet regulatory requirements your laboratory data must be..
ALCOA is a set of principles and guidelines used in the life sciences and other regulated spaces. Contemporaneous timely Original authentic Accurate correct Meaning..
Caliber Products Alcoa Plus Compliance For Pharma Data Integrity
ALCOA is a set of principles and guidelines used in the life sciences and other regulated spaces The acronym which has expanded over the years. Ensure data integrity through ALCOA Plus As defined by FDA guidance to meet regulatory requirements your laboratory data must be. ALCOA and ALCOA Plus Principles for Data Integrity ALCOA was an tool to implement the data integrity in pharmaceutical manufacturing facility. The principles outlined in ALCOA ALCOA and ALCOA support efforts toward data integrity and include ensuring that data is attributable and traceable. The ALCOA principles or ALCOA are a set of 9 data integrity principles introduced by the FDA and are central to GxP operation..
Comments